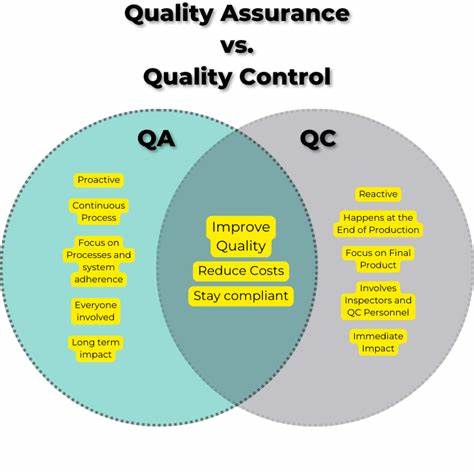
Quality Control (QC) and Quality Assurance (QA) are essential components of ensuring product quality in pharmaceutical manufacturing.
Quality Control (QC):
QC focuses on testing and evaluation to ensure product conformity to specifications.
Steps:
1. Sampling: Collect representative samples from production batches.
2. Testing: Conduct physical, chemical, and microbiological tests.
3. Inspection: Visually examine products for defects.
4. Data analysis: Evaluate test results against specifications.
5. Release: Approve or reject products based on test results.
Quality Assurance (QA):
QA encompasses a broader range of activities to ensure quality is built into the manufacturing process.
Steps:
1. Risk assessment: Identify potential quality risks.
2. Standard Operating Procedures (SOPs): Develop and implement SOPs.
3. Training: Educate personnel on QA/QC procedures.
4. Audits: Conduct regular internal audits.
5. Corrective and Preventive Actions (CAPAs): Implement changes to address deviations.
6. Continuous improvement: Monitor and refine processes.
7. Documentation: Maintain accurate records.
Key QA/QC Activities:
1. Material control
2. Equipment calibration and maintenance
3. Process validation
4. Cleaning and sanitation
5. Personnel qualification and training
6. Deviation management
7. Change control
8. Supplier qualification
QA/QC Tools:
1. Statistical Process Control (SPC)
2. Total Quality Management (TQM)
3. Six Sigma
4. Failure Mode and Effects Analysis (FMEA)
5. Root Cause Analysis (RCA)
Regulatory Requirements:
1. FDA (21 CFR)
2. EMA (EU GMP)
3. ICH (Q7, Q9, Q10)
4. ISO 9001 (Quality Management)
By integrating QC and QA processes, pharmaceutical manufacturers ensure compliance with regulatory requirements and produce high-quality products that meet patient needs.
Benefits:
1. Improved product quality
2. Reduced errors and deviations
3. Enhanced patient safety
4. Increased regulatory compliance
5. Better supply chain management
6. Improved business reputation
Challenges:
1. Resource constraints
2. Complex regulatory requirements
3. Rapid technological changes
4. Global supply chain risks
5. Ensuring data integrity
By understanding and implementing effective QC and QA processes, pharmaceutical manufacturers can ensure the quality and safety of their products.
Hello